Predict the relative bond angles in ccl4 and nh3 – Predicting the relative bond angles in CCl4 and NH3 unveils the intriguing world of molecular geometry, where VSEPR theory takes center stage. Delve into the fascinating journey of understanding how electron-pair repulsion shapes the architecture of these molecules, influencing their properties and reactivity.
As we explore the molecular landscapes of CCl4 and NH3, we will uncover the reasons behind their distinct tetrahedral and trigonal pyramidal geometries. Through comparative analysis, we will unravel the factors that govern bond angle variations, providing valuable insights into the fundamental principles of molecular structure.
Overview of Bond Angles: Predict The Relative Bond Angles In Ccl4 And Nh3
Bond angles are the angles formed between the bonds connecting atoms in a molecule. They play a crucial role in determining the molecular geometry, which in turn affects the physical and chemical properties of the molecule.
VSEPR (Valence Shell Electron Pair Repulsion) theory is a widely used model for predicting bond angles based on the repulsion between electron pairs in the valence shells of atoms.
Molecular Geometry of CCl4
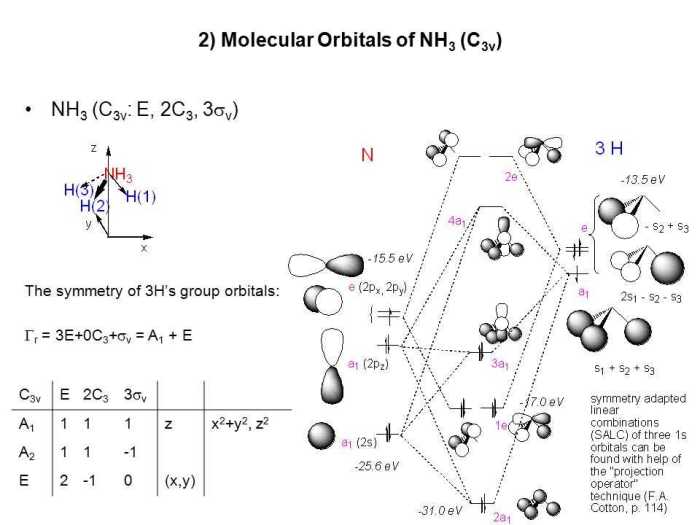
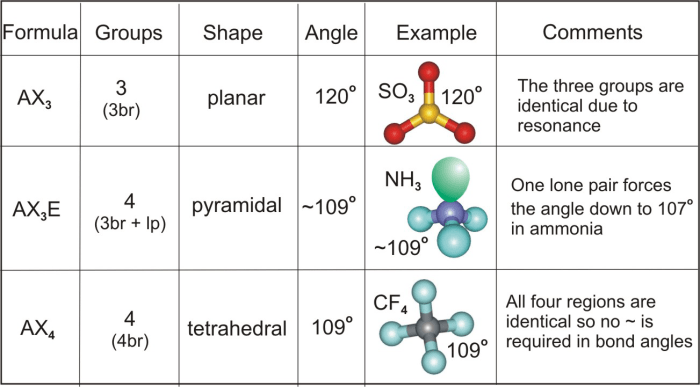
According to VSEPR theory, CCl4 has a tetrahedral molecular geometry. This is because the carbon atom in CCl4 has four electron pairs, which are arranged in a tetrahedral shape to minimize electron-pair repulsion.
In a tetrahedral geometry, the bond angles are all equal to 109.5 degrees.
Molecular Geometry of NH3, Predict the relative bond angles in ccl4 and nh3
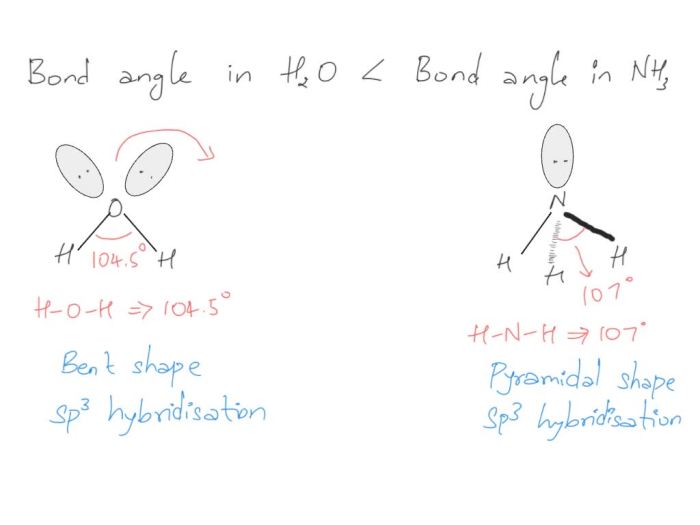
NH3 has a trigonal pyramidal molecular geometry. This is because the nitrogen atom in NH3 has four electron pairs, one of which is a lone pair. The lone pair repels the bonding electron pairs more strongly than the bonding electron pairs repel each other, resulting in a trigonal pyramidal geometry.
In a trigonal pyramidal geometry, the bond angles are not all equal. The bond angle between the three hydrogen atoms is approximately 107 degrees, while the bond angle between the nitrogen atom and each hydrogen atom is approximately 109.5 degrees.
Comparison of Bond Angles
The bond angles in CCl4 and NH3 are different because of the different molecular geometries of the two molecules. CCl4 has a tetrahedral geometry with all bond angles equal to 109.5 degrees, while NH3 has a trigonal pyramidal geometry with bond angles of approximately 107 degrees and 109.5 degrees.
The difference in bond angles affects the physical and chemical properties of the two molecules. For example, CCl4 is a nonpolar molecule, while NH3 is a polar molecule.
| Molecule | Molecular Geometry | Bond Angles |
|---|---|---|
| CCl4 | Tetrahedral | 109.5 degrees |
| NH3 | Trigonal Pyramidal | ~107 degrees, ~109.5 degrees |
Applications of Bond Angle Predictions
Bond angle predictions are used in various fields of chemistry, including:
- Predicting the molecular geometry of molecules
- Understanding the physical and chemical properties of molecules
- Designing new molecules with specific properties
For example, bond angle predictions have been used to design drugs that target specific proteins and to develop new materials with improved properties.
User Queries
What is the significance of bond angle predictions?
Bond angle predictions provide crucial insights into molecular geometry, influencing molecular properties such as polarity, reactivity, and intermolecular interactions.
How does VSEPR theory aid in predicting bond angles?
VSEPR theory considers electron-pair repulsion to predict the arrangement of atoms around a central atom, providing a framework for determining bond angles.
What factors contribute to the difference in bond angles between CCl4 and NH3?
The presence of lone pairs on nitrogen in NH3 introduces additional electron-pair repulsion, resulting in a smaller bond angle compared to CCl4, which has no lone pairs.