Draw cis-1-ethyl-3-methylcyclohexane in its lowest energy conformation, an exploration into the molecular intricacies of this cyclohexane derivative. This endeavor delves into the fascinating realm of conformational analysis, revealing the factors that govern the stability and behavior of this organic compound.
Understanding the conformational preferences of cis-1-ethyl-3-methylcyclohexane is crucial for comprehending its reactivity, physical properties, and potential applications. By examining the interplay of steric hindrance, ring strain, and dipole-dipole interactions, we gain insights into the molecular dynamics that shape this versatile molecule.
Structural Analysis of cis-1-ethyl-3-methylcyclohexane
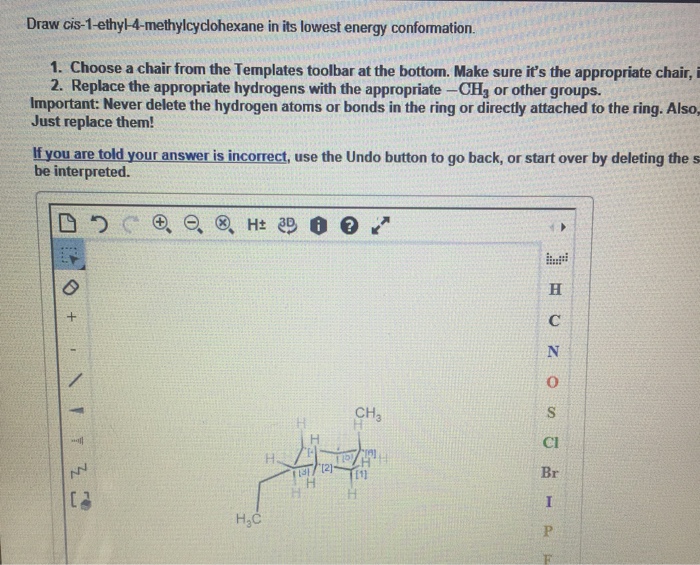
cis-1-ethyl-3-methylcyclohexane is a cyclohexane derivative with an ethyl group and a methyl group attached to the same side of the ring. The molecular structure can be described as a six-membered carbon ring with two substituents, an ethyl group (-CH2CH3) at carbon 1 and a methyl group (-CH3) at carbon 3. The “cis” designation indicates that the two substituents are on the same side of the ring, facing each other.
In cyclohexane rings, the concept of cis and trans isomers arises due to the ring’s flexibility. Cis isomers have both substituents on the same side of the ring, while trans isomers have them on opposite sides. This difference in spatial arrangement can significantly impact the molecule’s properties and reactivity.
Structural Representation, Draw cis-1-ethyl-3-methylcyclohexane in its lowest energy conformation
| Carbon Number | Substituent | Position | Stereochemistry |
|---|---|---|---|
| 1 | Ethyl (-CH2CH3) | Axial | cis |
| 3 | Methyl (-CH3) | Equatorial | cis |
Conformational Analysis
Conformational analysis involves studying the different spatial arrangements of atoms within a molecule, known as conformations. These conformations arise due to the rotation around single bonds. In cis-1-ethyl-3-methylcyclohexane, the ethyl and methyl groups can adopt different orientations relative to the cyclohexane ring.
The lowest energy conformation is the one in which the bulky ethyl group occupies an equatorial position, while the smaller methyl group is in an axial position. This conformation minimizes steric hindrance and ring strain, making it the most stable arrangement.
Factors Influencing Conformation: Draw Cis-1-ethyl-3-methylcyclohexane In Its Lowest Energy Conformation
The stability of different conformations is influenced by several factors:
- Steric hindrance:Bulky groups prefer equatorial positions to avoid unfavorable interactions with adjacent hydrogen atoms.
- Ring strain:Certain conformations introduce strain into the cyclohexane ring, increasing the energy of the molecule.
- Dipole-dipole interactions:Polar groups can interact through dipole-dipole forces, influencing the preferred conformation.
In cis-1-ethyl-3-methylcyclohexane, the equatorial ethyl group experiences less steric hindrance than the axial ethyl group. Additionally, the equatorial methyl group minimizes ring strain compared to the axial methyl group.
Spectroscopic Techniques for Conformational Analysis
Nuclear magnetic resonance (NMR) spectroscopy is a powerful tool for conformational analysis. By analyzing the chemical shifts and coupling constants of different protons, it is possible to determine the relative orientations of atoms within a molecule.
In the case of cis-1-ethyl-3-methylcyclohexane, NMR data can reveal the axial and equatorial positions of the ethyl and methyl groups. Other spectroscopic techniques, such as IR and UV-Vis spectroscopy, can also provide information about the molecular conformation.
Applications of Conformational Analysis

Conformational analysis plays a crucial role in drug design and development. Understanding the preferred conformations of drug molecules can help optimize their efficacy and minimize side effects.
By considering the conformational preferences of drug molecules, scientists can design drugs that bind more effectively to target proteins and avoid interactions with non-target molecules. This knowledge can lead to the development of more potent and selective drugs with reduced toxicity.
Question Bank
What is the significance of conformational analysis in understanding molecular behavior?
Conformational analysis provides insights into the three-dimensional arrangement of atoms within a molecule, which influences its reactivity, physical properties, and biological activity. By understanding the preferred conformations of a molecule, we can better predict its behavior in various environments.
How does steric hindrance affect the conformation of cis-1-ethyl-3-methylcyclohexane?
Steric hindrance refers to the repulsive interactions between bulky groups within a molecule. In cis-1-ethyl-3-methylcyclohexane, the ethyl and methyl groups experience steric hindrance when they are oriented towards each other, leading to a higher energy conformation. The lowest energy conformation minimizes these steric interactions.
What spectroscopic techniques can be used to determine the conformation of cis-1-ethyl-3-methylcyclohexane?
Nuclear magnetic resonance (NMR) spectroscopy is a powerful tool for conformational analysis. By analyzing the chemical shifts and coupling constants of the hydrogen atoms, we can determine the relative orientations of different groups within the molecule and identify the most stable conformation.