Relative mass and the mole pogil – Delving into the fascinating realm of chemistry, we encounter two fundamental concepts: relative mass and the mole. These concepts are essential for comprehending the composition and behavior of matter, providing a gateway to unraveling the secrets of chemical reactions and quantitative analysis.
Relative mass, a cornerstone of chemistry, quantifies the mass of an atom or molecule relative to a standard, allowing us to compare the masses of different substances. The mole, the SI unit of amount, serves as a bridge between the microscopic and macroscopic worlds, connecting the number of particles to their mass.
Definition of Relative Mass and the Mole
Relative mass is a dimensionless quantity that expresses the mass of an atom or molecule relative to a standard reference. The standard reference is the carbon-12 isotope, which is assigned a relative mass of exactly 12. The relative mass of an element is the average mass of its naturally occurring isotopes, weighted according to their abundance.
The mole is the SI unit of amount. It is defined as the amount of substance that contains as many elementary entities as there are atoms in 0.012 kilograms of carbon-12. The elementary entities can be atoms, molecules, ions, or electrons.
Calculating Relative Mass
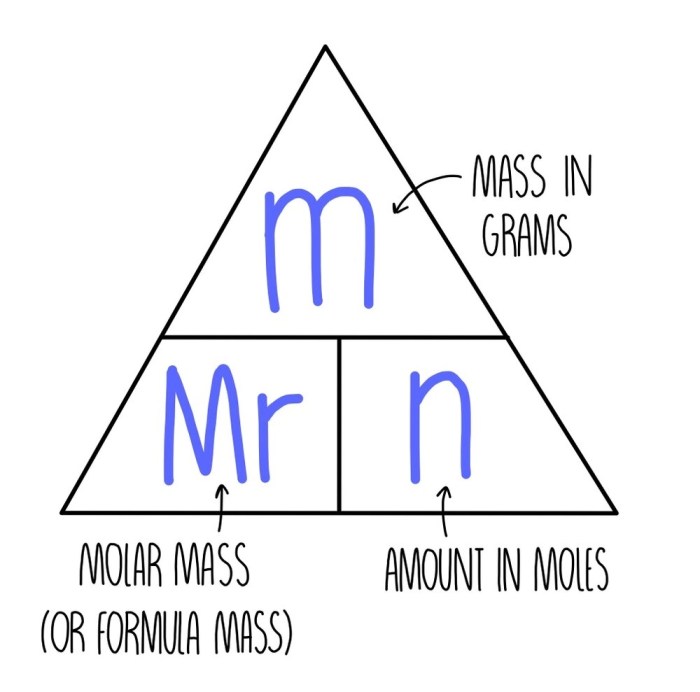
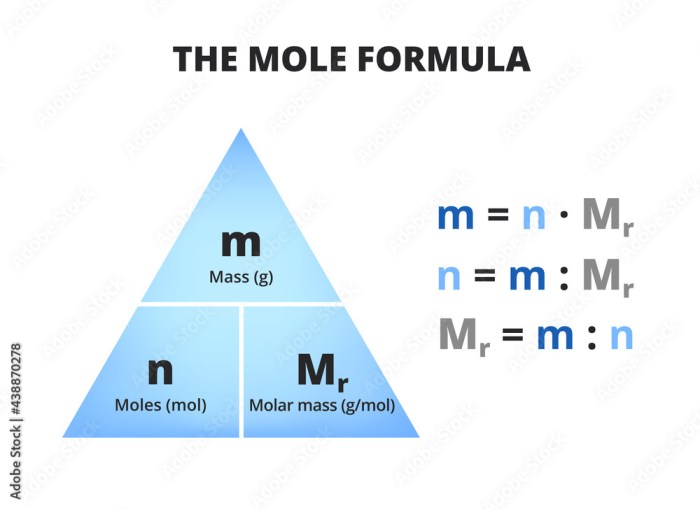
The relative mass of an element or compound can be calculated by dividing its mass by the mass of one mole of carbon-12.
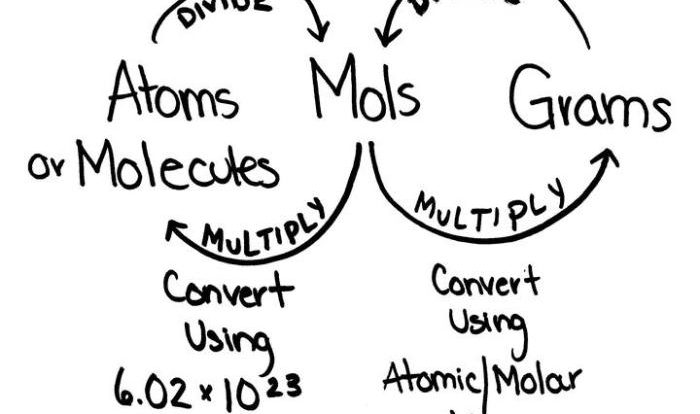
Avogadro’s Number and the Molar Mass: Relative Mass And The Mole Pogil
Avogadro’s number is the number of elementary entities in one mole of a substance. It is equal to 6.022 × 10 23.
The molar mass of a compound is the mass of one mole of that compound. It is calculated by adding the relative masses of the constituent elements.
Table of Molar Masses
The following table lists the molar masses of some common elements and compounds:
| Element | Molar Mass (g/mol) |
|---|---|
| Hydrogen | 1.008 |
| Carbon | 12.011 |
| Nitrogen | 14.007 |
| Oxygen | 15.999 |
| Sodium | 22.990 |
| Magnesium | 24.305 |
| Aluminum | 26.982 |
| Silicon | 28.085 |
| Phosphorus | 30.974 |
| Sulfur | 32.066 |
Empirical and Molecular Formulas
The empirical formula of a compound gives the simplest whole-number ratio of the elements in the compound. The molecular formula gives the actual number of atoms of each element in a molecule of the compound.
Determining Empirical Formula
The empirical formula of a compound can be determined from its elemental composition. The elemental composition can be determined by elemental analysis.
Determining Molecular Formula, Relative mass and the mole pogil
The molecular formula of a compound can be determined by using the molar mass and the empirical formula. The molecular formula is a multiple of the empirical formula.
Chemical Reactions and Stoichiometry
Stoichiometry is the study of the quantitative relationships between reactants and products in chemical reactions. The stoichiometric coefficients in a balanced chemical equation give the mole ratios of the reactants and products.
Using Stoichiometry
Stoichiometry can be used to calculate the amounts of reactants and products in a chemical reaction. It can also be used to determine the limiting reactant in a reaction.
FAQ Section
What is the significance of relative mass in chemistry?
Relative mass enables the comparison of the masses of different atoms and molecules, providing insights into their composition and structure.
How is the mole defined, and what is its relationship to relative mass?
The mole is the SI unit of amount, defined as the quantity of substance containing as many elementary entities as there are atoms in 0.012 kilograms of carbon-12. Its numerical value is equal to the relative mass of a substance.
Can you provide an example of calculating the relative mass of a compound?
To calculate the relative mass of a compound, multiply the relative mass of each element by the number of atoms of that element in the compound’s formula, and then add the results together.